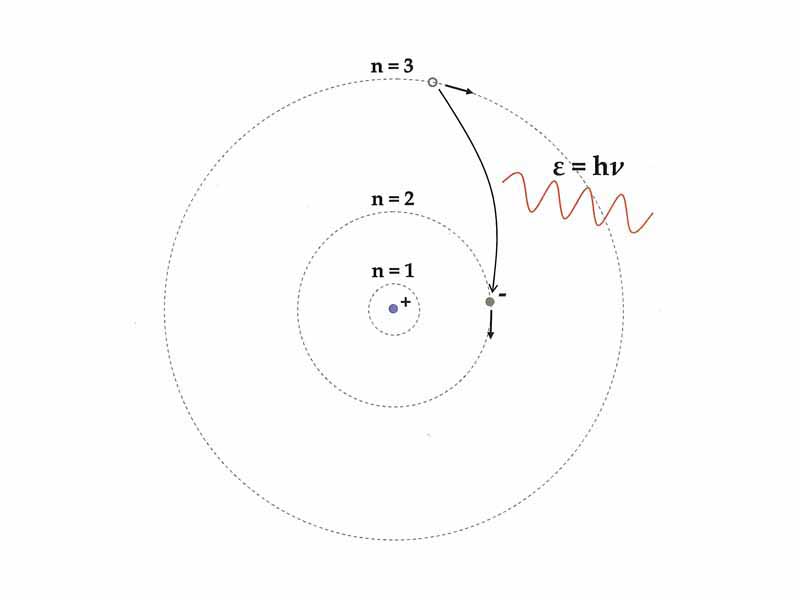
Niels Bohr’s 1913 quantum model of the atom, which incorporated an explanation of Johannes Rydberg's 1888 formula, Max Planck’s 1900 quantum hypothesis, i.e. that atomic energy radiators have discrete energy values (? = h?), J.J. Thomson’s 1904 plum pudding model, Albert Einstein’s 1905 light quanta postulate, and Ernest Rutherford's 1907 positive atomic nucleus discovery.
Click this LINK to visit the original image and attribution information. Right click on the image to save the 800px teaching JPEG.