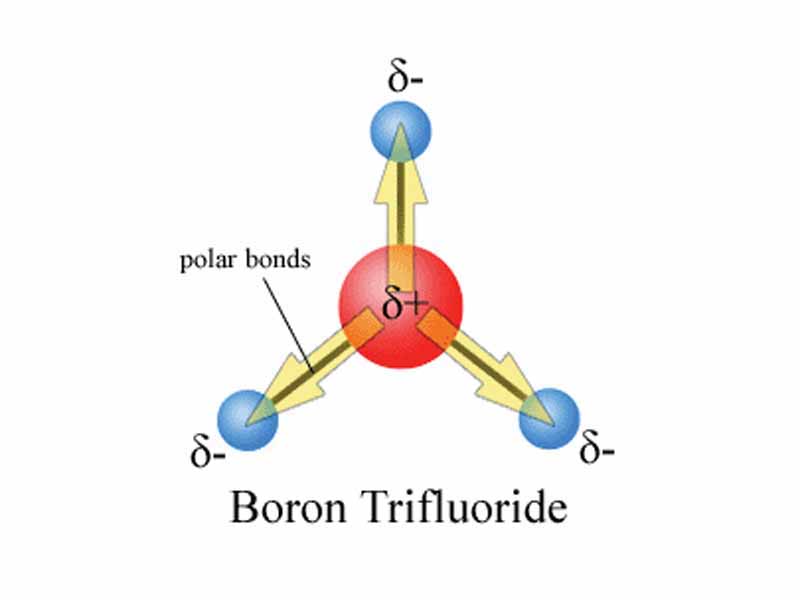
Diagram showing the net effect of symmetrical polar bonds (direction of yellow arrows show the migration of electrons) within boron trifluoride cancelling out to give a net polarity of zero. ?- shows an increase in negative charge and ?+ shows an increase in positive charge.
Click this LINK to visit the original image and attribution information. Right click on the image to save the 800px teaching JPEG.